Operational Excellence & Quality

Scroll
Kaizen and Lean Thinking at Work
We apply Kaizen principles across our operations, empowering every team member to identify and implement small, meaningful changes that drive long-term improvement. Whether it’s resolving process inefficiencies or improving turnaround times, continuous improvement is embedded in our daily routines.
Our use of Lean manufacturing methods helps eliminate waste, resolve bottlenecks, and ensure that every step in our processes adds value. From logistics to process design, we focus on what matters most: delivering high-quality products efficiently and reliably.
-
Long-term
improvement
-
Empowering
every team member
-
Eliminate
waste, resolve bottlenecks
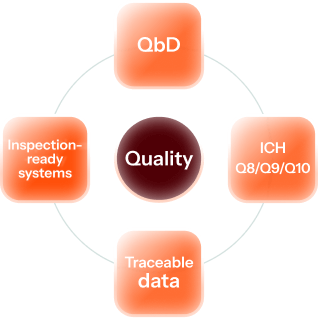
A Culture Built on Quality
Our teams are trained to think critically, ask questions, and challenge assumptions, and this fosters a culture of ownership and scientific integrity. Whether scaling a new formulation or managing a commercial campaign, we approach every lot with the same attention to detail.
Partnering with Accountability
Operational excellence means more than efficiency; it means showing up for our partners with urgency, open communications, and follow-through. From proactive project communication to responsive troubleshooting, Codis is built to deliver the confidence, control, and transparency your program deserves.
Contact UsDiscover Codis
Explore how Codis applies its expertise in spray drying, microencapsulation, continuous granulation, final dosage forms, and functional products to meet your formulation and manufacturing needs.
Codis Insights & Updates
Stay current with the latest in particle engineering science and Codis articles and resources.
Connect with us to explore how our particle engineering, commercial spray drying, formulation, and advanced manufacturing capabilities deliver high-quality, dependable supply for even the most challenging products.