Microencapsulation
Scroll
Advanced Microencapsulation Technology
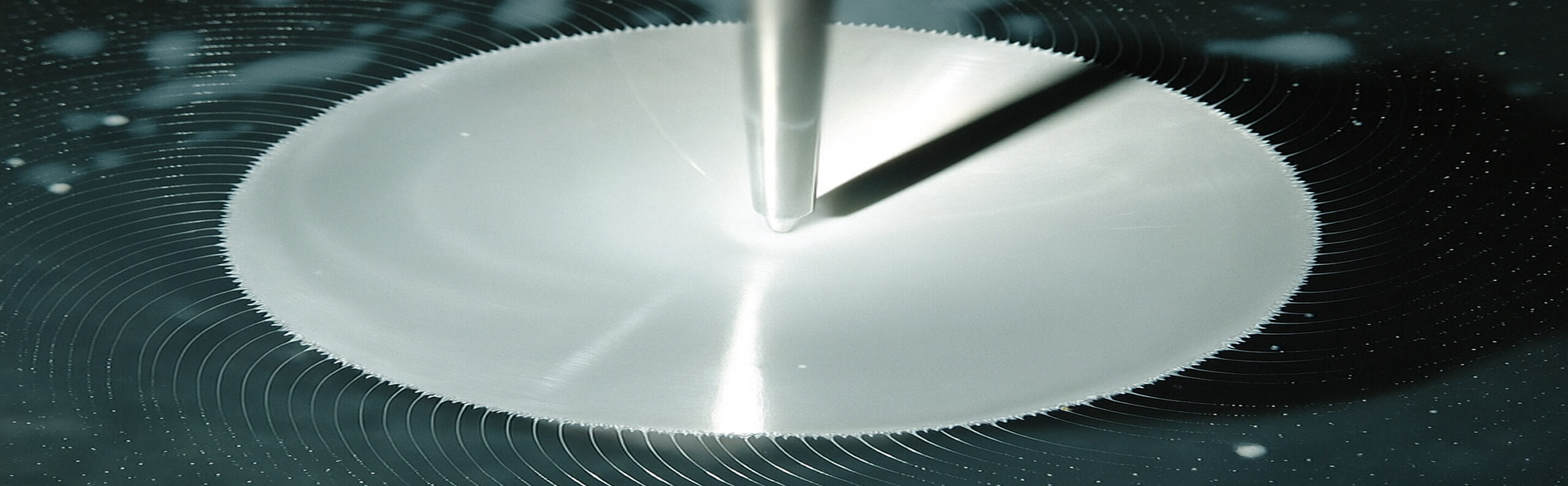
Codis’ microencapsulation technology utilizes a scalable, industry-proven spinning disc process to embed a variety of ingredients, such as vitamins, minerals, and pharmaceutical APIs, within a hydrophobic vegetable fatty acid matrix. This lipid barrier shields the active from direct contact with taste buds and olfactory receptors, delivering effective taste and odor masking. The encapsulation also protects sensitive actives from environmental degradation, enhancing stability and shelf life. In addition, the coating can enable controlled or delayed release, allowing for targeted delivery and optimal performance across a wide range of oral dosage forms.
The Codis Microencapsulation Advantage
Our proprietary microencapsulation process delivers performance and flexibility unmatched by conventional techniques:
Solvent-free processing
ensures an environmentally friendly, pure product with no residual solvents
Robust, uniform coatings
protect particles during downstream processing without rupturing
Exceptional taste and odor masking
enhances the palatability of nutritional and pharmaceutical ingredients
Improved stability
safeguards sensitive actives from environmental degradation
Free-flowing, directly compressible granules
improve tableting efficiency and throughput
Excellent mouthfeel and dissolution
for optimal patient and consumer experience
Controlled- or delayed-release
options support diverse delivery profiles
Flavor-infused coatings
enable creative product development
Microencapsulation Applications
Our microencapsulation technology enables taste masking, odor control, stability enhancement, and tailored release profiles across a wide range of nutritional, OTC, and prescription applications.
-
Nutritional
Transform the sensory experience of fortified products while enhancing stability and performance. Nutritional applications for our microencapsulation technology include:
- Chewable multivitamin and mineral tablets
- Nutrient-fortified powdered beverage mixes
- Energy and nutrition bars
- Fortified pre-mix blends
-
Prescription (Rx) & OTC
Deliver better-tasting, patient-friendly medicines that maintain potency and meet rigorous quality standards. Applications include:
- Pediatric and adult chewable tablets
- Pediatric and adult orally dissolving tablets (ODTs)
- Pediatric and adult flavored orally dissolving powders (ODPs)
Codis Microencapsulation At-a-Glance
Facility Highlights
-
Manufacturing Facility:
St Louis, Missouri, USA
-
Compliance:
The facility and its processes comply with US FDA 21 CFR Parts 210/211, 110/111, and 117, as well as EU drug certification standards.
-
Standard Products:
Our full line of Descote® vitamins and minerals ; MicroMask® APIs
-
Custom Products:
We develop and manufacture customized, proprietary client compounds in the vitamin, mineral, OTC, and pharmaceutical markets. Contact us to get started.
Discover Codis
Explore how Codis applies its expertise in spray drying, microencapsulation, continuous granulation, final dosage forms, and functional products to meet your formulation and manufacturing needs.
Codis Insights & Updates
Stay current with the latest in particle engineering science and Codis articles and resources.
Connect with us to explore how our particle engineering, commercial spray drying, formulation, and advanced manufacturing capabilities deliver high-quality, dependable supply for even the most challenging products.